Medical device testing – Always one step ahead.
As a DAkkS accredited testing laboratory for medical devices, we support manufacturers, medical doctors and experts in the field of medical device testing, damage analysis as well as research and development of medical devices.
We provide among other test services: strength and wear tests of joint endoprostheses, osteosynthesis products, trauma products, surgical instruments and skin adhesives and patch products according to a broad range of standards or customized test procedures.
IMA Dresden gladly advises you on the required tests for your device to receive approval. We also support you with the development of test procedures beyond standardized testing – based on scientific studies and databases.
Our portfolio
Range of testing of the medical devices
Range of services
Since 1995 we have successfully performed medical device test services on implant products.
| Wear testing | Materialography (microstructure analysis, etc.) |
| Fatigue testing (tension, pressure, torsion, shear, etc.) | Damage analysis |
| Corrosion testing | Materials and coatings |
| Contact area/stress test (surface pressure) | Sample preparation |
| Simulation and strength assessment | Determination of metal ion concentration |
| Artificial aging | PE particle analysis – all in one hand: filter preparation and SEM analysis |
| Mechanical and physical tests on PE (crystallinity, degree of crosslinking, Charpy, etc.) |
Joint implants
Joint implants are loaded for up to ten million cycles in the anatomically correct position under physiologic conditions in our laboratories. With our accredited test programs, we simulate the lifetime of an implant.
Please accept functionality cookies to display content.
Our joint simulators, each with eight stations, allow wear tests to be carried out on knee, ankle and shoulder endoprostheses in accordance with standards, as well as wear tests under more stringent conditions.
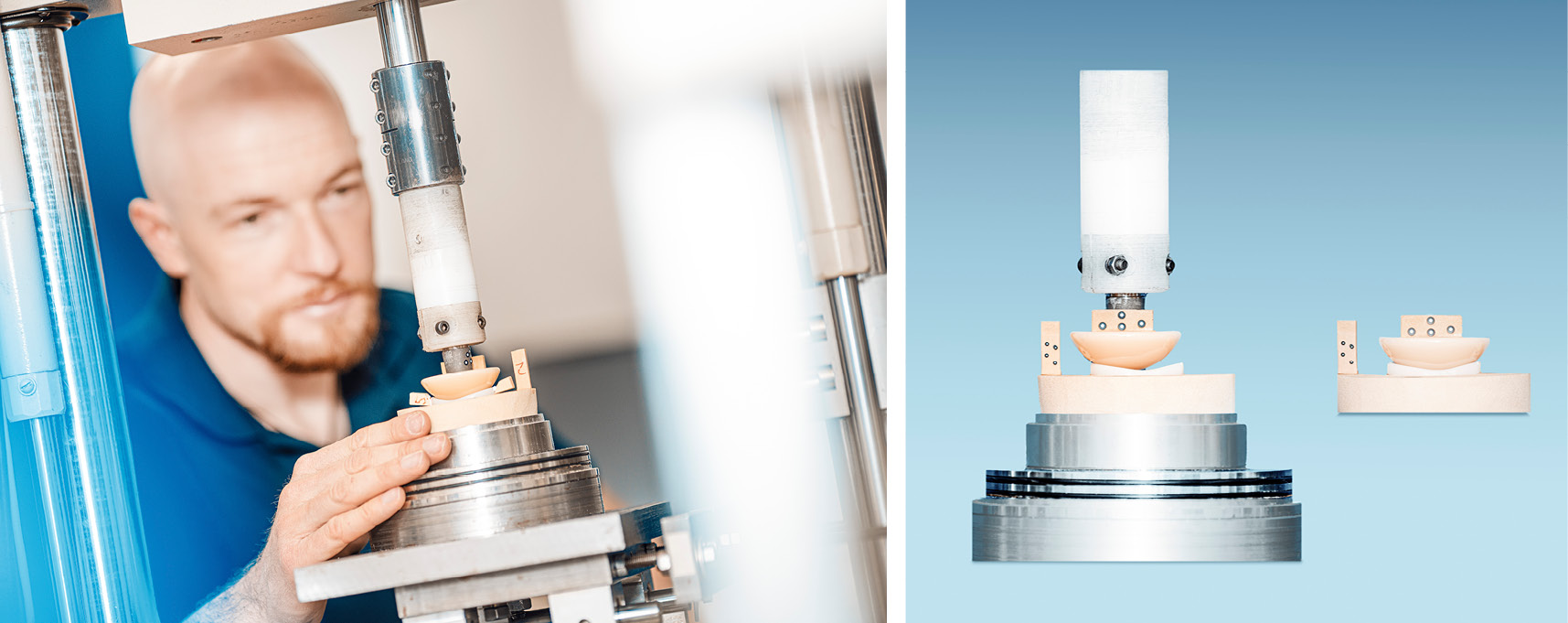
Hip Joint Implants
The technical documentation for hip joint implants requires the verification of several device characteristics on the basis of standardized test procedures. Our laboratory facilities enable us to conduct these tests in with highest precision.
Standards
Test standard overview in the field of hip joint implant
Knee joint implants
The large number of different types of knee endoprosthetics with regard to fitting type, fixation, bearing couple, design and degree of coupling require defined test series for the approval process.
Standards
Test standard overview in the field of knee joint implant
Shoulder joint implants
We have added in-house test instructions to the standardized test procedures for even more meaningful results based on scientific publications and databases. Thus, we cover anatomical and reverse designs. Edge loading is of particular interest for these endoprostheses.
Standards
Test standard overview in the field of shoulder joint implants
Ankle Joint Implants
Since 2019 we perform standardized wear tests on ankle joint implants. These are also realized reliably and in accordance with standards in our modern wear simulators.
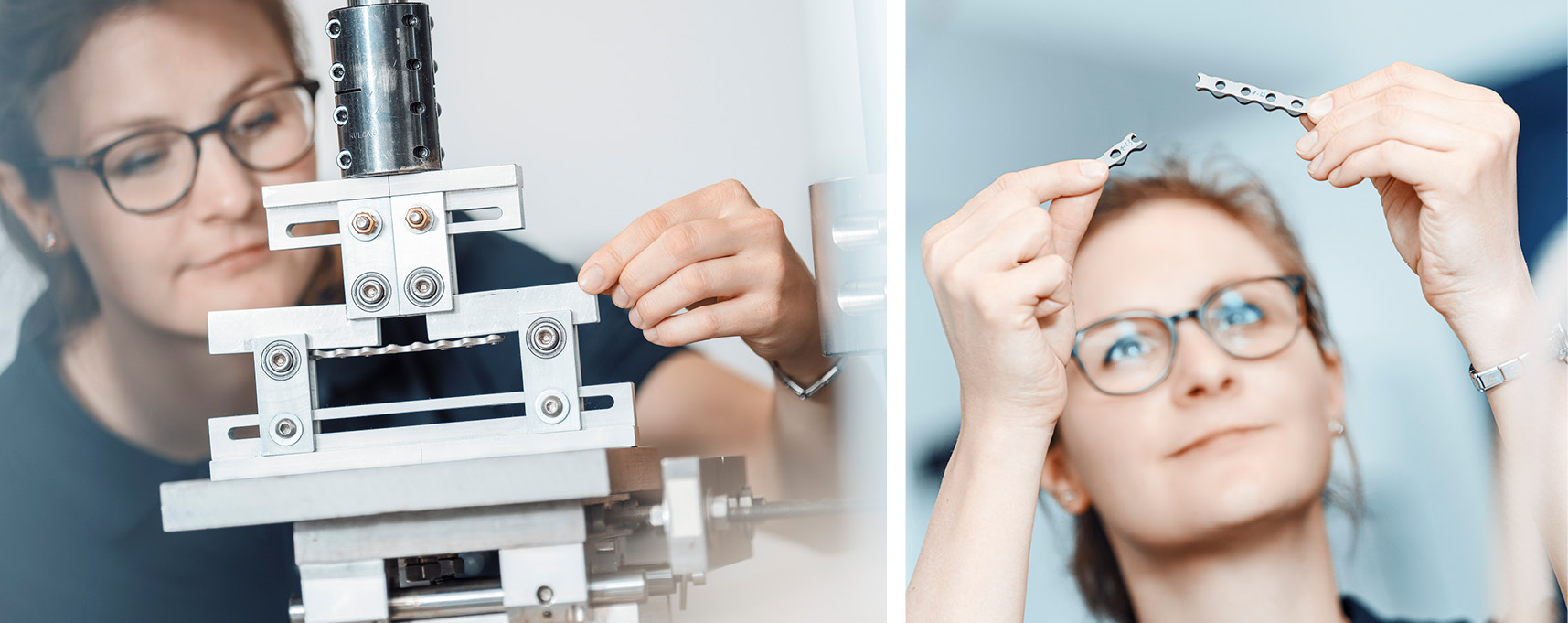
Osteosynthesis implants
Among other things, bone screws must resist defined torsional stresses. The assessment of the flexural strength is of particular importance for bone plates. In case of failure, you can rely on the expertise in our materialography laboratory.
We test for you:
- Bone screws
- Bone plates
- Intramedullary nails
Standards
Test standard overview in the field of osteosynthesis implants
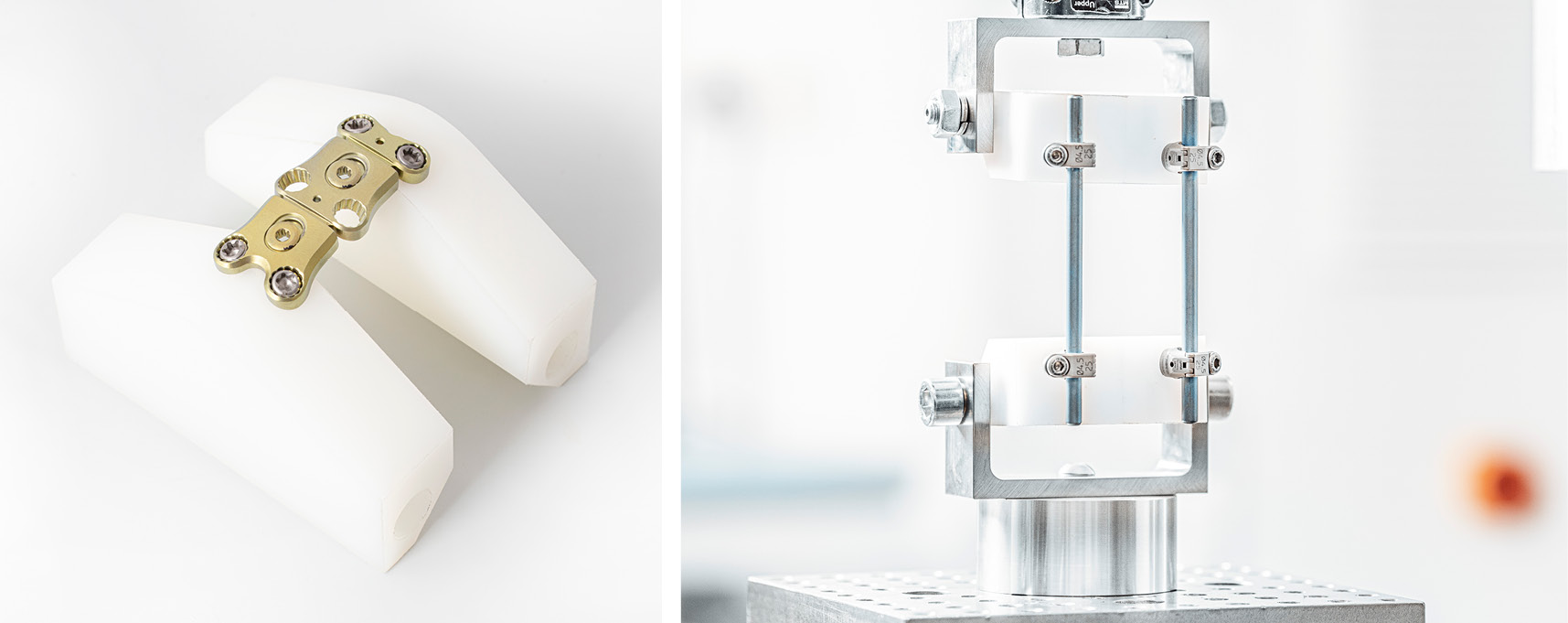
Spinal implants
In addition to the tension, compression and torsion loading tests, also combined loading scenarios on spinal systems can be carried out. The components can be tested statically as well as dynamically in our laboratories according to current standards.
We test for you:
- Spinal fixators (screw-rod system)
- Fusion implants
- Artificial discs
Standards
Test standard overview in the field of spinal implants
Adhesives for skin
Wound or tissue adhesives are an alternative to the conventional wound closure through surgical sutures. To ensure safe use, the adhesive properties must be determined and the wound closure strength demonstrated before clinical use.
Our laboratory is accredited for various skin adhesive tests according to DIN EN ISO/IEC 17025.
Surgical instruments
We also test impact-loaded surgical instruments for their durability and check for any functional impairment through the testing process. The conversion is carried out by means of cyclic impact test procedures on a specially developed test fixture. The produced load profiles are validated through extensive clinical measurement data sets.
Methods of endurance and load tests for instruments of different designs and adaptations are described in an IMA Dresden test specification.
Range of services:
- Dynamic testing of orthopedic instruments
- Testing for fatigue strength in endurance and load increase tests
- Including impact and setting instruments of the joint types shoulder, hip and knee
Materials and coatings
In addition to design, the use of suitable materials or coatings as the basis for long-term success after implantation. Materials tests and analyses on material samples as well as on finished implants and surgical instruments ensure an increasingly reliable product for the patient.
Standards
Overview of testing standards for physical tests on metals
Overview of testing standards for physical tests on plastics
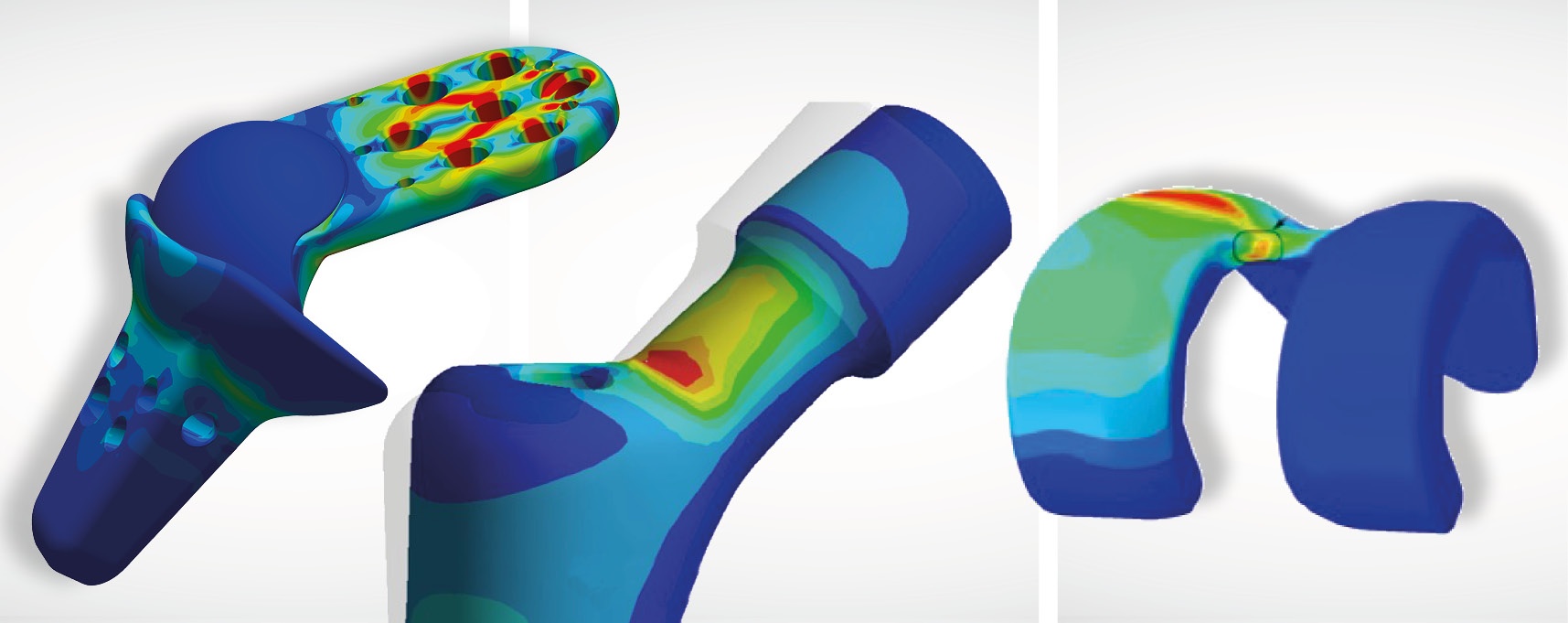
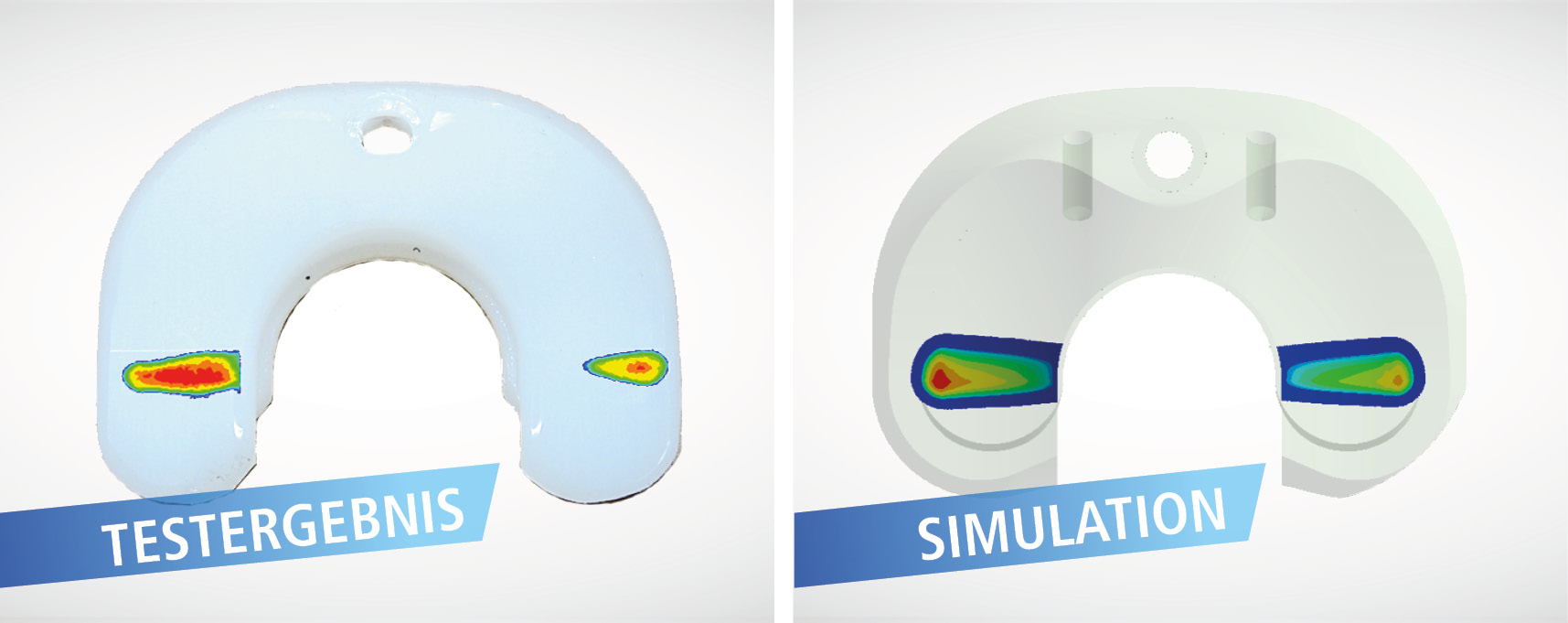
Simulation and strength assessments
With calculated strength assessment and versatile simulation capabilities, IMA Dresden supports you in optimizing your designs to make device developments marketable faster.
Range of services:
- Simulation of contact situations
- Simulation of interference fits
- Simulation of hyperelastic materials and plastics
- Lifetime analyses of the structure
- Determination of worst-case geometry (e.g. according to ASTM F2996, ASTM F3161), which lead to a shortening of the test times and to a cost reduction in the experimental verifications.
Our mission is the structural and cost optimization of your medical device already in the development stage.
Standards
Test standard overview in the field of simulatio
Materialography and damage analysis
We document the damage for you and determine the cause of it. In systematic damage analyses we can utilize various microscopic and material analytical methods to get to the bottom of material failure.
Read more here: Materialography Damage analysis